In the old days (5 years ago) an emerging Biotech would not invest in a manufacturing suite - there is a mature environment of contract manufacturers for both small molecule and biologics. And a GMP facility is very expensive to build and operate.
But this has all changed. As new powerful and proprietary techniques combining genomics, biological synthesis, and personalized medicine emerge, contract manufacturing is not an option - many emerging Biotechs want to or must manufacture their therapy candidates. If only they had the money...
The economics have changed, too - some of our Biotech customers have raised $200m, $500m, $1.2B in the capital markets - in part because a manufacturing suite to deliver tailored or personalized mRNA, CAR-T or other advanced biologics are expensive to build. This then is the third integration priority of the emerging Biotech - manufacturing integration.
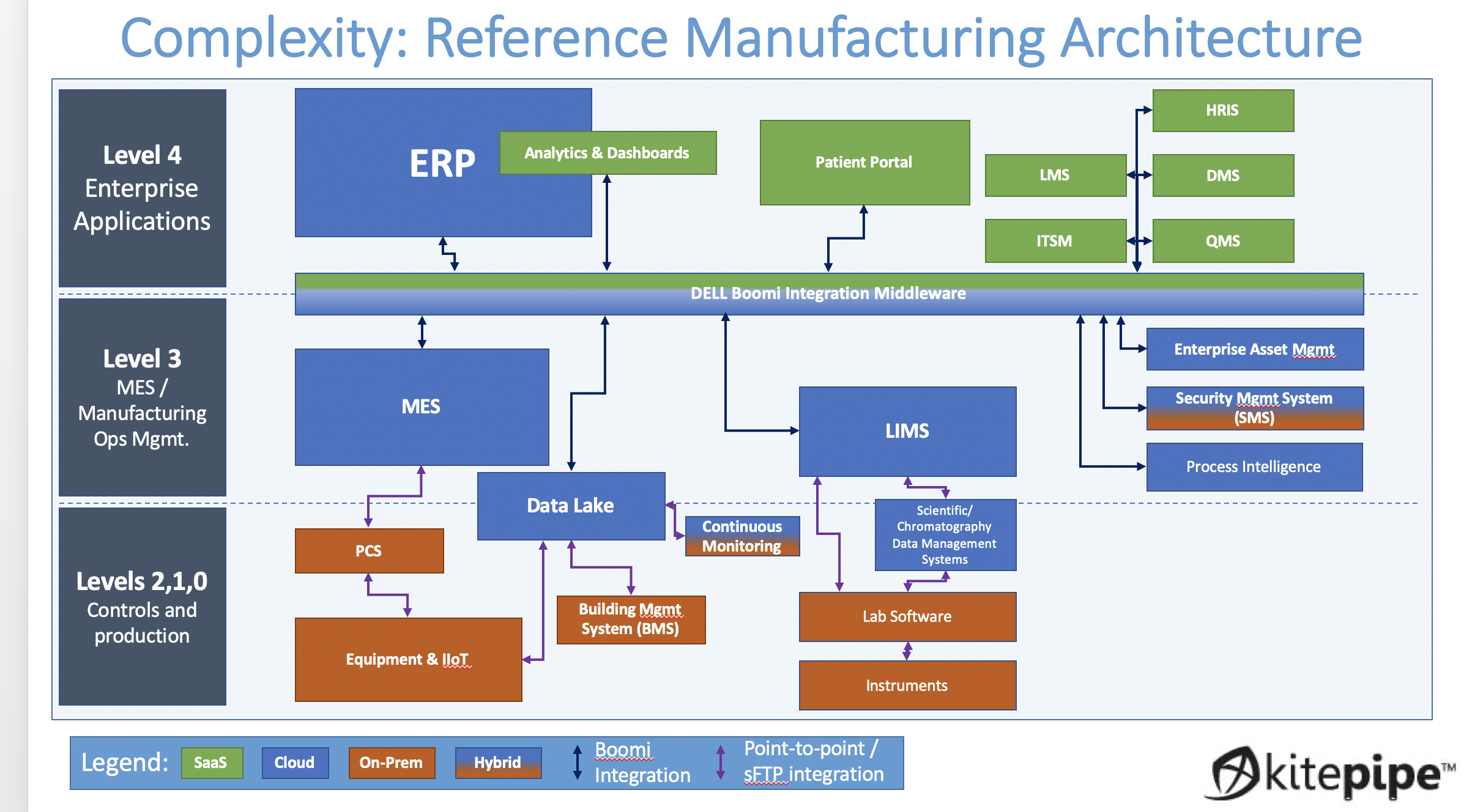
A modern biotech manufacturing suite is insanely complex. Inventory, processes, orders, testing, status, training, specification, and quality must all be managed, and often each has its own proprietary platform. In the old days (5 years ago) the pace of progress allowed the printing out, wet signing, and scanning of paper documents as a way to move data across these many platforms. But for many obvious reasons, a "Paperless" operation with pervasive digital integration is now the goal.